Two Health Data Research UK studies find only small elevated risk of blood clots following AstraZeneca COVID-19 vaccination
22 February 2022
Two large studies carried out independently in the UK and supported by Health Data Research UK and the BHF Data Science Centre found a slight increase in risk of intracranial thromboses in some populations after the AstraZeneca COVID-19 vaccine.
There is a slightly elevated risk of intracranial thrombosis events following vaccination with the AstraZeneca ChAdOx1-S COVID vaccine, according to two new studies publishing February 22nd in PLOS Medicine.
The first paper was led by William Whiteley of the University of Edinburgh and the CVD-COVID-UK consortium, with colleagues from the Longitudinal Health and Wellbeing COVID-19 National Core Study, analysed the electronic health records of 46 million adults in England. The second paper, by Steven Kerr of the University of Edinburgh, UK, and colleagues from the Swansea University Health Data Research UK team used a dataset of 11 million adults in England, Scotland, and Wales.
Cases of thromboses—when a blood clot blocks a vein or artery—have been reported after vaccination with the Astra Zeneca ChAdOx1-S COVID-19 vaccine. However, the rates of common venous and arterial events, including stroke, myocardial infarction, deep vein thrombosis and pulmonary embolism, are hard to measure based on case reports alone.
In the first study, Whiteley et al. analysed linked NHS England data from over 46 million adults in England, from the beginning of the COVID-19 vaccination programme on 8 December 2020 until 18 March 2021. Researchers looked for episodes of blood clots in the arteries or veins, and of low levels of platelets (blood cells that help with clotting) in adults before COVID-19 vaccination, after COVID-19 vaccination and in those not vaccinated at all. They considered separately those under the age of 70 years and those aged 70 years and over, and those who received a first vaccine of either the Oxford-AstraZeneca or Pfizer-BioNTech vaccine.
The research team used NHS Digital’s Trusted Research Environment (TRE) Service for England, which ensures that data is secure. To protect privacy, all identifiers (such as names, addresses and NHS numbers) were removed.
Importantly, this study minimised the chance that something other than the vaccine could be causing associations of vaccines with blood clots, by taking into account a comprehensive range of other factors known to impact on the incidence of clotting events. These included age, sex, ethnicity, deprivation level, previous medical history and medications.
The research was conducted by members of the CVD-COVID-UK consortium, a National Institute for Health Research (NIHR) and British Heart Foundation (BHF) flagship project led by the BHF Data Science Centre at Health Data Research UK. The CVD-COVID-UK consortium is a collaborative group of more than 260 members across over 50 institutions across the UK, working together with patient and public contributors to understand the relationship between COVID-19 and cardiovascular diseases such as heart attack and stroke in the UK population.
The research was also conducted by researchers from the Longitudinal Health and Wellbeing COVID-19 National Core Study, and supported by the British Heart Foundation, the Stroke Association, and the Data and Connectivity COVID-19 National Core Study.
William Whiteley, project lead at the University of Edinburgh, said: “Because of its very large size, this research study has provided precise results on the risks of rare blood clotting events and of low platelet levels following COVID-19 vaccination. We were able to show that these risks occur only in people under 70 years old with the Oxford-AstraZeneca vaccine and that the increase in risk is extremely small – no more than a few people per million vaccinated.
Cathie Sudlow, Director of the BHF Data Science Centre said: “This study illustrates the importance of linking and analysing data from several different healthcare settings for a very large population. Including data from 46 million adults meant we could pick up very rare clotting events, while linking data across different healthcare settings in the safe setting of the NHS Digital Trusted Research Environment meant we could take into account a very broad range of factors apart from vaccination that affect the risk of blood clots.
“The BHF Data Science Centre is delighted to have been able to support this collaborative, team science effort involving members of the public as well as researchers from many UK institutions.”
Prof Jonathan Sterne from the University of Bristol, who is co-lead of the Longitudinal Health and Wellbeing COVID-19 National Core Study, said “The COVID-19 vaccines have been crucial to the UK and international response to the pandemic, but like all vaccines they have rare side effects. I hope that, by putting the small risk of serious harms in the context of the huge benefits of vaccination, our work can help reassure people who are concerned about these side effects.
Dr Richard Francis, Head of Research at the Stroke Association said: “We are relieved to see that this research has shown that COVID-19 vaccines are unlikely to cause stroke except in fewer than 1-3 people in a million.
“Unfortunately, we already know that catching the virus without being vaccinated significantly increases your risk of a stroke. This research will help people to understand how their overall risk of stroke is affected by getting vaccinated. We look forward to the findings of research into why catching the virus increases your risk of stroke and how to prevent this.”
Professor Sir Nilesh Samani, Medical Director at the British Heart Foundation, said:
“The results of this latest analysis should provide reassurance to anyone who might have delayed getting their covid-19 vaccine due to concerns about side effects. It shows that the risk of developing dangerous blood clots is much higher if you get Covid-19. That’s why it’s important to avoid Covid-19, and the best way to do that is to stay up-to-date with your vaccine doses.
“This study also illustrates the enormous benefits of access to routinely collected healthcare data for research to answer questions of real importance and value to patients and the public. Our single NHS system is uniquely powerful to enable this, and we set up the BHF Data Science Centre with this precise ambition in mind”
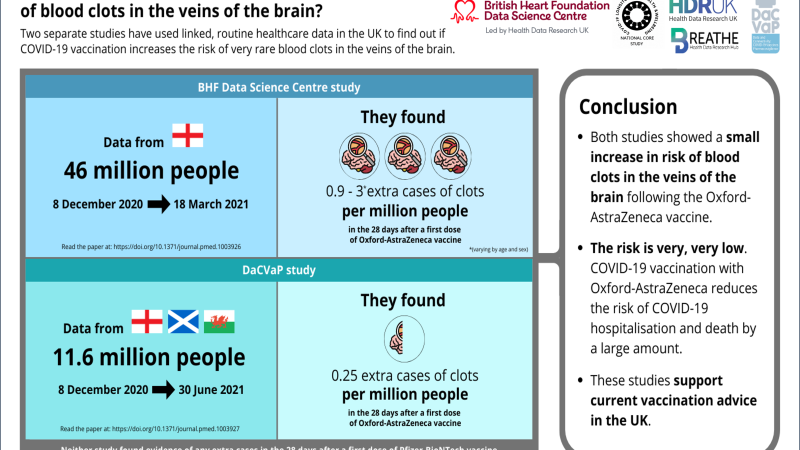
In the second study, Kerr et al., linked data spanning December 2020 through June 2021 from multiple sources—including primary care, secondary care, mortality and virological testing—for more than 11 million people in England, Scotland and Wales. They compared the rate of cerebral venous sinus thrombosis (CVST) events—a rare type of blood clot in the brain—in the 90 days prior to vaccination and the four weeks following a first dose of the AstraZeneca ChAdOx1-S vaccine or the Pfizer BNT162b2 vaccine.
The authors observed a small elevated risk of CVST events following vaccination with ChAdOx1-S, equivalent to one additional event per 4 million people vaccinated, which was approximately twice as high as before vaccination. The study found no association between the BNT162b2 and CVST.
“This evidence may be useful in risk-benefit evaluations for vaccine-related policies, and in providing quantification of risks associated with vaccination to the general public,” the authors say.
The authors of both studies caution that the low number of overall events of CVST and other subtypes of thromboses, even in large cohorts, makes precise estimates of the risks difficult. They plan to carry out future studies to include other vaccines as well as second and booster vaccinations.
Kerr says, “In this analysis using data from England, Scotland and Wales, we found a roughly two-fold increased risk of a rare form of blood clot in the brain following the Oxford-AstraZeneca vaccine, but we did not see any increased risk for the Pfizer vaccine. We used a novel method that allowed us to do the analysis across several countries without them having to share person-level data with each other, and we hope to take advantage of this in future to allow greater collaboration across the UK.”
Health Data Research UK are the UK’s national institute for health data research, and were involved in the collaborative partnerships that enabled access to the de-identified data for both studies.
Andrew Morris, Director and CEO of Health Data Research UK said, “HDR UK are pleased to have supported two papers published today which independently report a minor elevated risk of blood clotting events following the AstraZeneca vaccine, but not the Pfizer vaccine.
“At the start of the vaccine roll out, validating reports of an increased risk of blood clotting events were of critical policy-making importance. Given the rarity of these events, large datasets were required to accurately assess the safety the vaccine and guide its ongoing roll out.
“As the UK’s national health data research institute, HDR UK and the BHF Data Science Centre worked with the research groups to rapidly facilitate secure access to large data sets and provide evidence both at huge scale, and across the UK, to inform Government policy and put the public’s mind at ease on the risks and benefits of the vaccine.”
You can discover the datasets used in this research on the HDR UK Innovation Gateway

Visit the Gateway
A common entry point for researchers to discover and request access to UK health datasets for life saving research.