Precision antimicrobial prescribing: safeguarding patient outcomes and preserving future efficacy

This project aims to improve antimicrobial prescribing decisions by analysing linked data to optimise antibiotic choice for patients based on their individual history and clinical characteristics.
The Challenge
Antibiotic drugs are a crucial part of modern medicine, but rising levels of resistance and a lack of new drugs in development mean that we must use existing drugs carefully. Resistance to antibiotic drugs, known as antimicrobial resistance (AMR), causes 700,000 deaths annually worldwide, predicted to rise to 10 million annually by 2050 with a total economic burden over this period of up to $100 trillion. Clinicians often lack information about their patients’ antibiotic history, and the drug susceptibility of patients’ infections meaning patients often finish treatment without a microbiological diagnosis.
The Solution
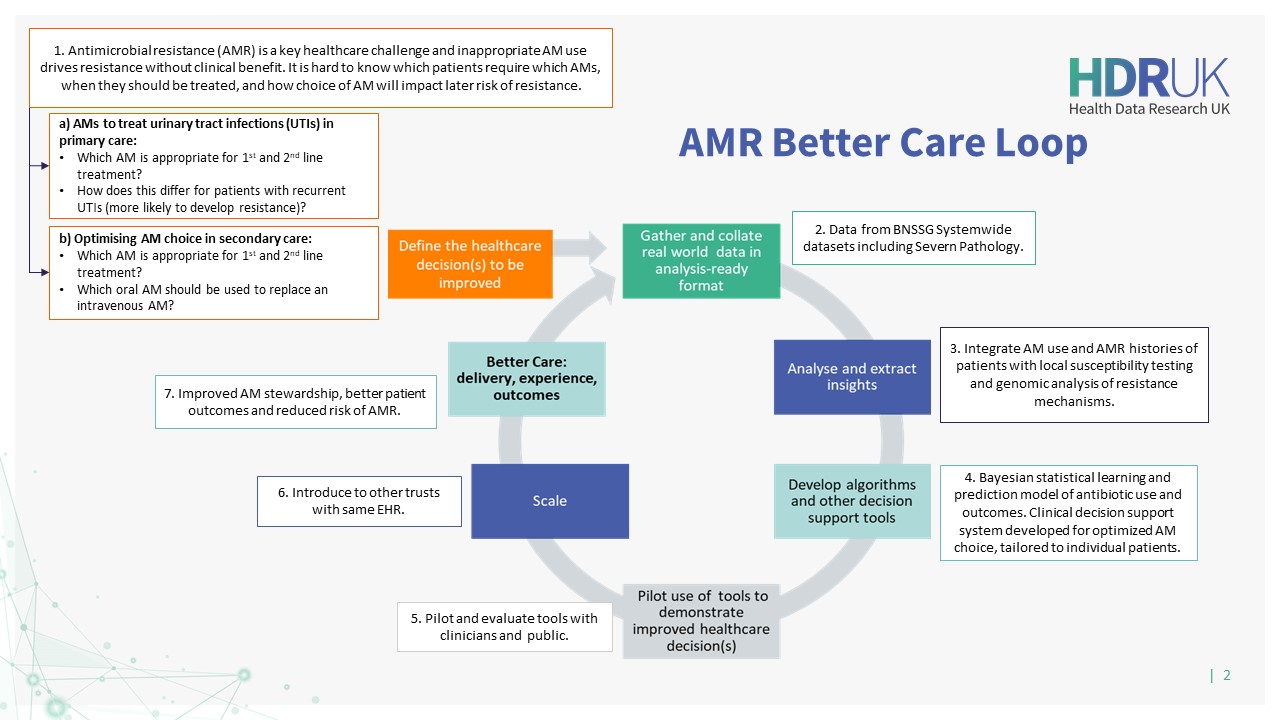
Information about antibiotic use and AMR is held within different databases, for example, in general practices, hospitals, laboratories and pharmacies, across the health system. The team will analyse data from the Bristol, North Somerset and South Gloucestershire (BNSSG) Systemwide dataset of linked primary care, secondary care and laboratory records to optimise antibiotic choice for patients based on their individual history and clinical characteristics, as well as their personal, and the population’s, risk of resistance.
Building on their combined expertise in data science, data linkage and genomic analysis, the team will model resistance rates over time and the association of resistance with antibiotic use and a broad range of patient demographics and other metadata.
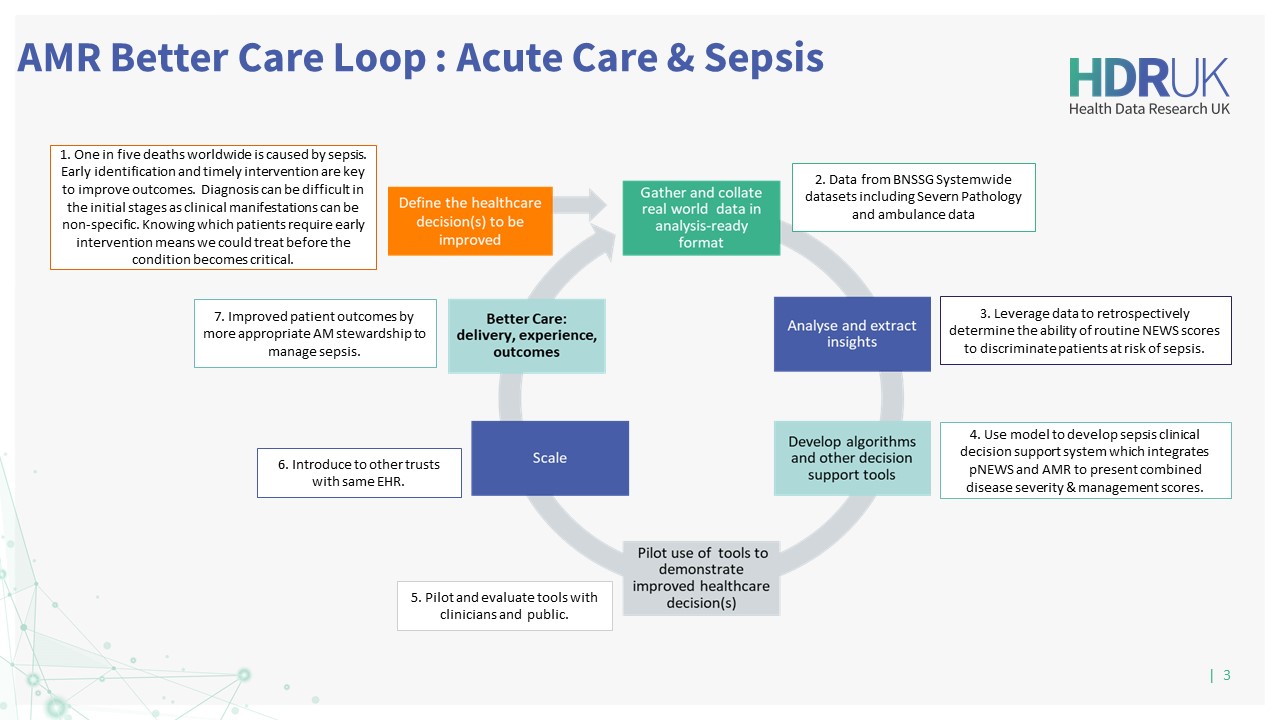
Co-creating with partnering clinicians, the team will use the model to develop a prediction tool to optimise decision-making in clinical pathways across three Better Care Loops in primary care, secondary care, and the identification and management of sepsis in critical care.
The Impact and Outcomes
This project will provide a Clinical Decision Support System for antimicrobial (AM) prescribing across primary, secondary, and critical care, informed by historical AM use and resistance data at the individual and population-level. It will generate predictions guiding clinicians to optimise AM stewardship answering: What antibiotic, when and in whom?
Targeted AM prescribing will reduce exposure to ineffective AMs and the associated side effects and provide earlier treatment with effective AM, ultimately improve patient outcomes and supporting the global fight against AMR.
Better Care Loops
Project Team
Prof Andrew Dowsey, Professor of Population Health Data Science, Population Health Sciences and Bristol Veterinary School, University of Bristol
Dr Katy Turner, Associate Professor in Infectious Disease Epidemiology, Bristol Veterinary School, University of Bristol